Learning objectives / Learning outcomes / Specification link-up / Kerboodle
Students should learn:
· what crude oil is
· what an alkane is
· how to represent alkanes / Most students should be able to:
· recognise that crude oil is a mixture and state that it can be separated into fractions by distillation
· define and recognise simple alkanes write the correct chemical formula of an alkane represented by a structural formula.
· draw diagrams and write the formulae of simple alkanes when given named examples
· recall and use the formula CnH2n12 to give the formula of an alkane, when n is given. / Crude oil is a mixture of a very large number of compounds. [C1.4.1 a)]
A mixture consists of two or more elements or compounds not chemically combined together. The chemical properties of each substance in the mixture are unchanged. It is possible to separate the substances in a mixture by physical methods including distillation. [C1.4.1 b)]
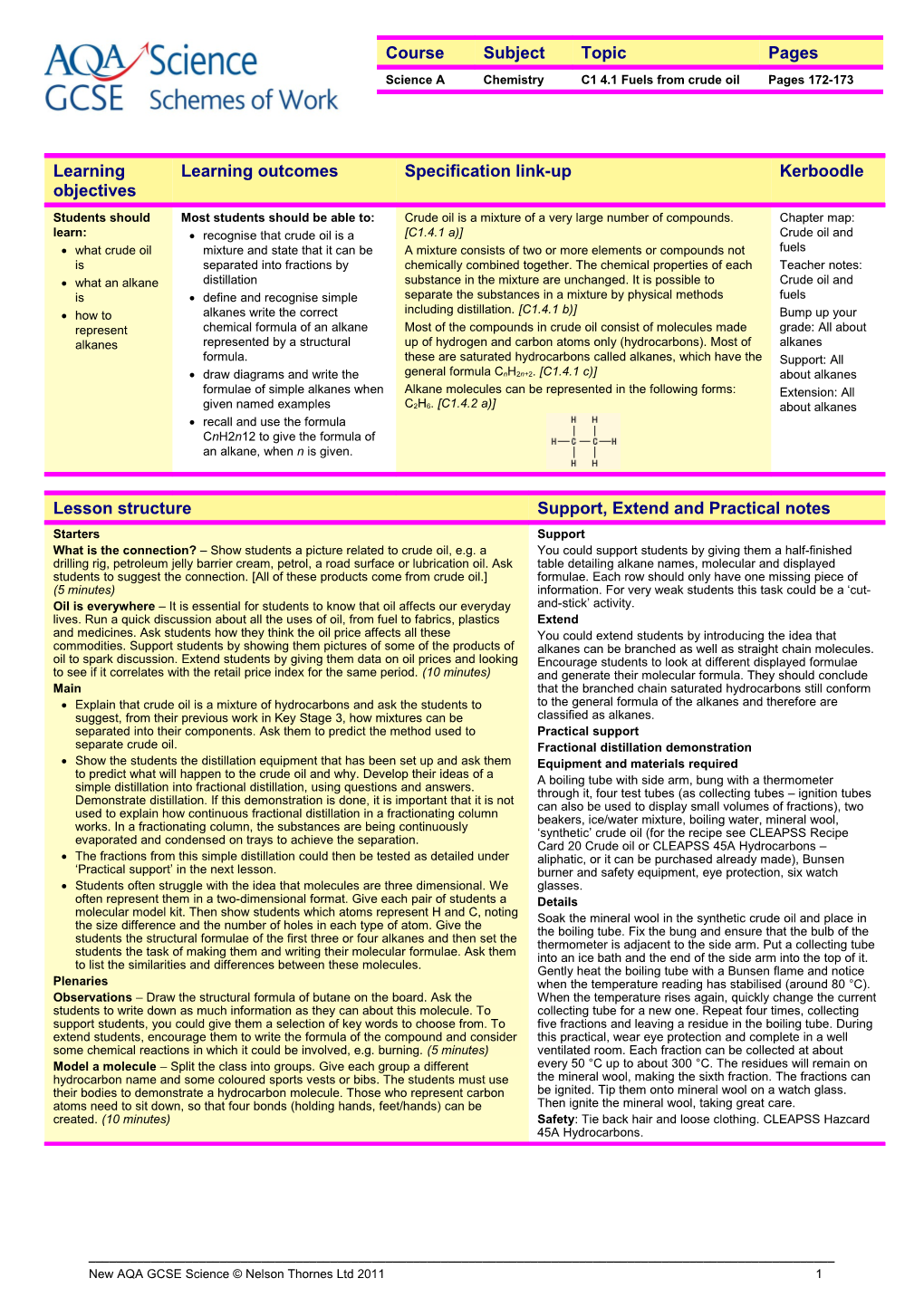
Most of the compounds in crude oil consist of molecules made up of hydrogen and carbon atoms only (hydrocarbons). Most of these are saturated hydrocarbons called alkanes, which have the general formula CnH2n+2. [C1.4.1 c)]
Alkane molecules can be represented in the following forms: C2H6. [C1.4.2 a)]
/ Chapter map: Crude oil and fuels
Teacher notes: Crude oil and fuels
Bump up your grade: All about alkanes
Support: All about alkanes
Extension: All about alkanes
Lesson structure / Support, Extend and Practical notes
Starters
What is the connection? – Show students a picture related to crude oil, e.g. a drilling rig, petroleum jelly barrier cream, petrol, a road surface or lubrication oil. Ask students to suggest the connection. [All of these products come from crude oil.] (5minutes)
Oil is everywhere – It is essential for students to know that oil affects our everyday lives. Run a quick discussion about all the uses of oil, from fuel to fabrics, plastics and medicines. Ask students how they think the oil price affects all these commodities. Support students by showing them pictures of some of the products of oil to spark discussion. Extend students by giving them data on oil prices and looking to see if it correlates with the retail price index for the same period. (10 minutes)
Main
· Explain that crude oil is a mixture of hydrocarbons and ask the students to suggest, from their previous work in Key Stage 3, how mixtures can be separated into their components. Ask them to predict the method used to separate crude oil.
· Show the students the distillation equipment that has been set up and ask them to predict what will happen to the crude oil and why. Develop their ideas of a simple distillation into fractional distillation, using questions and answers. Demonstrate distillation. If this demonstration is done, it is important that it is not used to explain how continuous fractional distillation in a fractionating column works. In a fractionating column, the substances are being continuously evaporated and condensed on trays to achieve the separation.
· The fractions from this simple distillation could then be tested as detailed under ‘Practical support’ in the next lesson.
· Students often struggle with the idea that molecules are three dimensional. We often represent them in a two-dimensional format. Give each pair of students a molecular model kit. Then show students which atoms represent H and C, noting the size difference and the number of holes in each type of atom. Give the students the structural formulae of the first three or four alkanes and then set the students the task of making them and writing their molecular formulae. Ask them to list the similarities and differences between these molecules.
Plenaries
Observations – Draw the structural formula of butane on the board. Ask the students to write down as much information as they can about this molecule. To support students, you could give them a selection of key words to choose from. To extend students, encourage them to write the formula of the compound and consider some chemical reactions in which it could be involved, e.g. burning. (5 minutes)
Model a molecule – Split the class into groups. Give each group a different hydrocarbon name and some coloured sports vests or bibs. The students must use their bodies to demonstrate a hydrocarbon molecule. Those who represent carbon atoms need to sit down, so that four bonds (holding hands, feet/hands) can be created. (10 minutes) / Support
You could support students by giving them a half-finished table detailing alkane names, molecular and displayed formulae. Each row should only have one missing piece of information. For very weak students this task could be a ‘cut-and-stick’ activity.
Extend
You could extend students by introducing the idea that alkanes can be branched as well as straight chain molecules. Encourage students to look at different displayed formulae and generate their molecular formula. They should conclude that the branched chain saturated hydrocarbons still conform to the general formula of the alkanes and therefore are classified as alkanes.
Practical support
Fractional distillation demonstration
Equipment and materials required
A boiling tube with side arm, bung with a thermometer through it, four test tubes (as collecting tubes – ignition tubes can also be used to display small volumes of fractions), two beakers, ice/water mixture, boiling water, mineral wool, ‘synthetic’ crude oil (for the recipe see CLEAPSS Recipe Card 20 Crude oil or CLEAPSS 45A Hydrocarbons – aliphatic, or it can be purchased already made), Bunsen burner and safety equipment, eye protection, six watch glasses.
Details
Soak the mineral wool in the synthetic crude oil and place in the boiling tube. Fix the bung and ensure that the bulb of the thermometer is adjacent to the side arm. Put a collecting tube into an ice bath and the end of the side arm into the top of it. Gently heat the boiling tube with a Bunsen flame and notice when the temperature reading has stabilised (around 80 °C). When the temperature rises again, quickly change the current collecting tube for a new one. Repeat four times, collecting five fractions and leaving a residue in the boiling tube. During this practical, wear eye protection and complete in a well ventilated room. Each fraction can be collected at about every 50 °C up to about 300 °C. The residues will remain on the mineral wool, making the sixth fraction. The fractions can be ignited. Tip them onto mineral wool on a watch glass. Then ignite the mineral wool, taking great care.
Safety: Tie back hair and loose clothing. CLEAPSS Hazcard 45A Hydrocarbons.
Course / Subject / Topic / Pages
Science A / Chemistry / C1 4.1 Fuels from crude oil / Pages 172-173
Learning objectives / Learning outcomes / Specification link-up / Kerboodle
Students should learn:
· that crude oil is separated into fractions using fractional distillation
· the properties of each fraction and how they relate to the size of the molecules
· which fractions make useful fuels and why. / Most students should be able to:
· state that crude oil is separated into fractions by fractional distillation
· list how the properties change from small chain fractions to long chain fractions
· state which fractions are useful fuels.
Some students should also be able to:
· explain the key steps involved in fractional distillation relate the trend in properties to molecular size. / The many hydrocarbons in crude oil may be separated into fractions, each of which contains molecules with a similar number of carbon atoms, by evaporating the oil and allowing it to condense at a number of different temperatures. This process is fractional distillation. [C1.4.2 b)]
Some properties of hydrocarbons depend on the size of their molecules. These properties influence how hydrocarbons are used as fuels. [C1.4.2 c)] / Practical: Investigating the fractions of crude oil
Lesson structure / Support, Extend and Practical notes
Starters
Distil the order – Students could try to put these key words about distillation in order: condense, mixture, separate, boil, heat. [Mixture, heat, boil, condense, separate.] (5 minutes)
Fuel list – Ask students to consider what they have used today that relies on a fuel. Ask various students to give their thoughts to the class. For example, transport (petrol, diesel and more recently autogas), heating (gas, oil), cooking (gas, charcoal for barbecues) or lighting (gas, oil). Students could be supported by showing them photographs to act as a stimulus. Extend students by asking them to explain how electricity is related to fuel use. (10minutes)
Main
· To contrast fractional distillation in a school lab with what happens in industry, students could watch a video on separation of crude oil such as Industrial Chemistry for Schools and Colleges (RSC).
· Then the students could be given a drawing of a fractionating column, to which they would add their own notes. To support students, this activity could be adapted into a cut-and-stick exercise, which gives the key points as words and diagrams on a piece of paper and the students assemble a poster.
· Often students do not know what a fraction of crude oil looks like. Ampoules of the different crude oil fractions could be shown to the students (available from BP: www.bpes.com).
· Give the students some string beads. Then ask them to cut or break links to give different lengths to represent the different fractions (each bead represents a carbon atom). Put these onto a demonstration table – one pile for each fraction.
· Show the students samples of different fractions (e.g. light a Bunsen burner or show a camping gas bottle, sample of octane, paraffin, lubricating oil and wax).
· Ask the students to comment on the colour, viscosity and state at room temperature. Then try to ignite some of each fraction and ask the students to note the flame colour and ease of ignition.
· Ask the students to compare the properties with the length of the molecule. This task could be written up in the form of a results table.
· Link here to ‘Controlled Assessment’ – relationships between variables.
Plenaries
Improvisation – Ask for volunteers to talk about a key word for 30 seconds without ‘erms’ or pauses. Ask the student to talk about hydrocarbons, fractions or viscosity – without any preparation! The volunteer is given a word and starts talking while the rest of the class listens. If there are any misconceptions, ask the other students to pick them out. Students could be supported by allowing them a few minutes of preparation time and working in small groups. Students could be extended by being asked to use a prop in their talk. (5 minutes)
Questions and answers – Give each student either a question or an answer on index cards. Ask the students to find their partners. (10 minutes) / Support
· If a teaching assistant is available, split the class in two. The teacher could demonstrate the properties of the different fractions, while the teaching assistant shows the ampoule (sealed glass container) samples of the fractions. Then rotate the groups.
· Students may need some support with remembering the different displayed formulae. You could display these on the board from small to larger. Then draw an arrow from small to large molecules. Ask students to state the trend – the colour darkens, viscosity increases and ease of lighting reduces.
Extend
Students should be encouraged to understand that there are forces of attraction between the molecules and it is these that have to be overcome and reformed as each molecule slides over others as the liquid is poured. Then encourage students to use this information to explain why larger molecules are more viscous. They could explain this model using a series of cartoons.
Practical support
Comparing fractions (demonstration)
Equipment and materials required
Three samples of different alkanes (flammable) to represent different fractions (choose fractions with very different hydrocarbon chain lengths such as hexane, paraffin and candle wax), eye protection, four watch glasses, evaporating dish, mineral wool, dropping pipette, heat-proof mat, matches, molecular model kits.
Details
Pour each alkane onto a separate watch glass, starting with the smallest carbon chain. Ask students to comment on the viscosity, then the colour. Students should conclude that the longer the hydrocarbon chain, the more viscous the liquid and the darker it is. Then, try lighting a small amount of the alkane soaked into mineral wool, in an evaporating dish. Ask students to comment on the ease of lighting and the colour of the flame. Students should conclude that the flame is ‘dirtier’ and the hydrocarbons are more difficult to light as the hydrocarbon’s length increases. You might wish to have molecular models of each of the fractions to aid students in making the link between hydrocarbon chain length and properties.
Safety: Ensure the stock bottles of alkanes are closed before lighting matches. CLEAPSS Hazcard 45B Paraffin – harmful.
Course / Subject / Topic / Pages
Science A / Chemistry / C1 4.2 Fractional distillation / Pages 174-175
Course / Subject / Topic / Pages
Science A / Chemistry / C1 4.3 Burning fuels / Pages 176-177
Learning objectives / Learning outcomes / Specification link-up / Kerboodle
Students should learn:
· the combustion products formed from the complete combustion of fuels
· the pollutants produced when we burn fuels. / Most students should be able to:
· write word equations for the complete combustion of hydrocarbons
· describe differences between incomplete and complete combustion
· list pollutants formed when we burn fuels.
Some students should also be able to:
· complete balanced symbol equations for the complete combustion of simple alkanes
· explain how nitrogen oxides, sulphur dioxide and particulates are produced during the combustion process. / Most fuels, including coal, contain carbon and/or hydrogen and may also contain some sulfur. The gases released into the atmosphere when a fuel burns may include carbon dioxide, water (vapour), carbon monoxide, sulfur dioxide and oxides of nitrogen. Solid particles (particulates) may also be released. [C1.4.3 a)]
New AQA GCSE Science Nelson Thornes Ltd 2011 10