Name:______Date:______Period:____
Acids and Base Bell Ringers – Show all Work
2/7/11 – Acids and Bases Introduction Notes
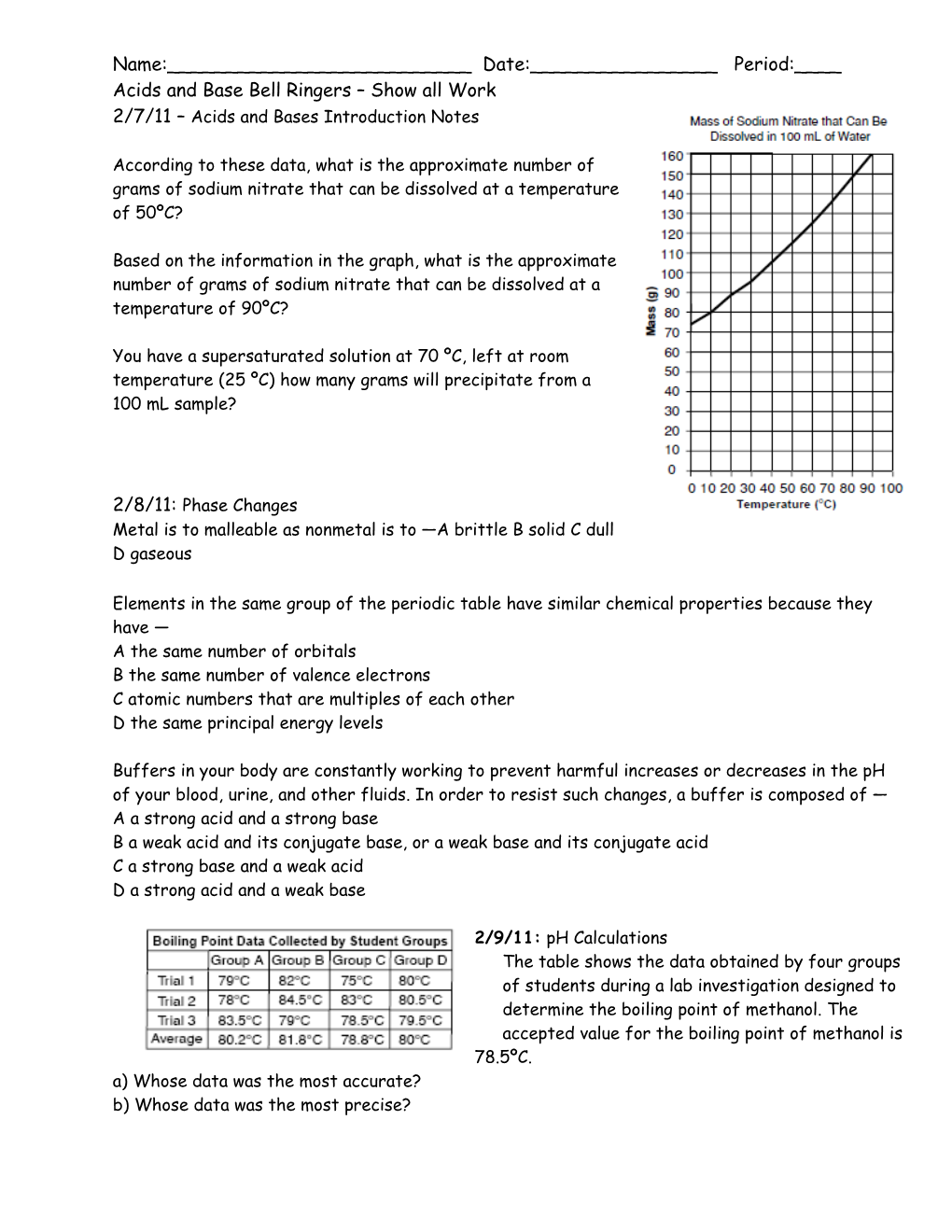
According to these data,what is the approximatenumber of grams of sodium nitrate that can bedissolved at a temperature of 50ºC?
Based on the information in the graph,what is theapproximate number of grams of sodium nitrate thatcan be dissolved at a temperature of 90ºC?
You have a supersaturated solution at 70ºC, left at room temperature (25ºC) how many grams will precipitate from a 100 mL sample?
2/8/11: Phase Changes
Metal is to malleable as nonmetal is to —AbrittleBsolidCdullDgaseous
Elements in the same group of the periodic tablehave similar chemical properties because they have —
Athe same number of orbitals
Bthe same number of valence electrons
Catomic numbers that are multiples of each other
Dthe same principal energy levels
Buffers in your body are constantly working toprevent harmful increases or decreases in the pH ofyour blood, urine, and other fluids. In order to resistsuch changes, a buffer is composed of —
A a strong acid and a strong base
B a weak acid and its conjugate base, or a weakbase and its conjugate acid
C a strong base and a weak acid
D a strong acid and a weak base
2/9/11:pH Calculations
The table shows the data obtained by four groups ofstudents during a lab investigation designed todetermine the boiling point of methanol. Theaccepted value for the boiling point of methanol is
78.5ºC.
a) Whose data was the most accurate?
b) Whose data was the most precise?
Which of these decreases as the pH of a solutionincreases?
A The basicity of a solution
B Number of hydrogen ions
C The value of Kw
D Number of hydroxide ions
The neutralization of a strong acid by a strong basealways involves the products —
A water and a salt
B an anion and a salt
C water and an ion
D a weak acid and a strong base
2/10/11
A student told the class that she takes 500 mg ofvitamin C every day. What is this mass expressed ingrams?
In the periodic table, a series of elements that isarranged in a horizontal row is called a —
A cluster
B family
C period
D group
2/11/11
Which of these phase changes does NOT involve theabsorption of heat energy?
A Boiling
B Condensation
C Melting
D Vaporization
Zinc is used as a coating on iron and steel to preventcorrosion. What is the mass, in grams, of 0.0650mol Zn?
Potassium nitrate, also known as saltpeter, is used inmatches. What is the percent by mass of potassium(K) in potassium nitrate (KNO3)?